Historical instruments
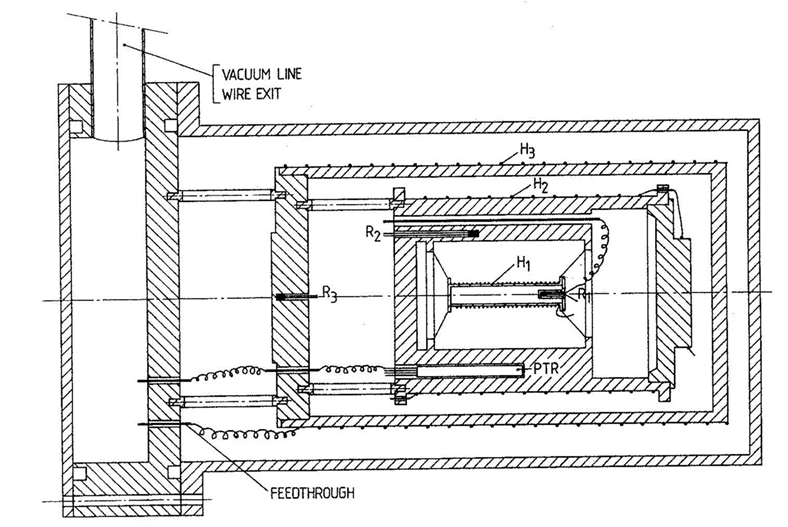
Before the current pASC-type calorimeters were constructed, an older generation of ASCs existed. These were heavy and complex instruments, difficult to operate and maintain, and yet, they provided unprecedented scientific results.
An example of such instrument is presented on the right. The complexity stems from the efforts to make the instrument truly adiabatic, by extensive wiring and construction and a complicated sample mounting.
To obtain a very controlled environment, several different temperature-controlled shields were implemented, and operation in vacuum was a requirement.
The pASC concept
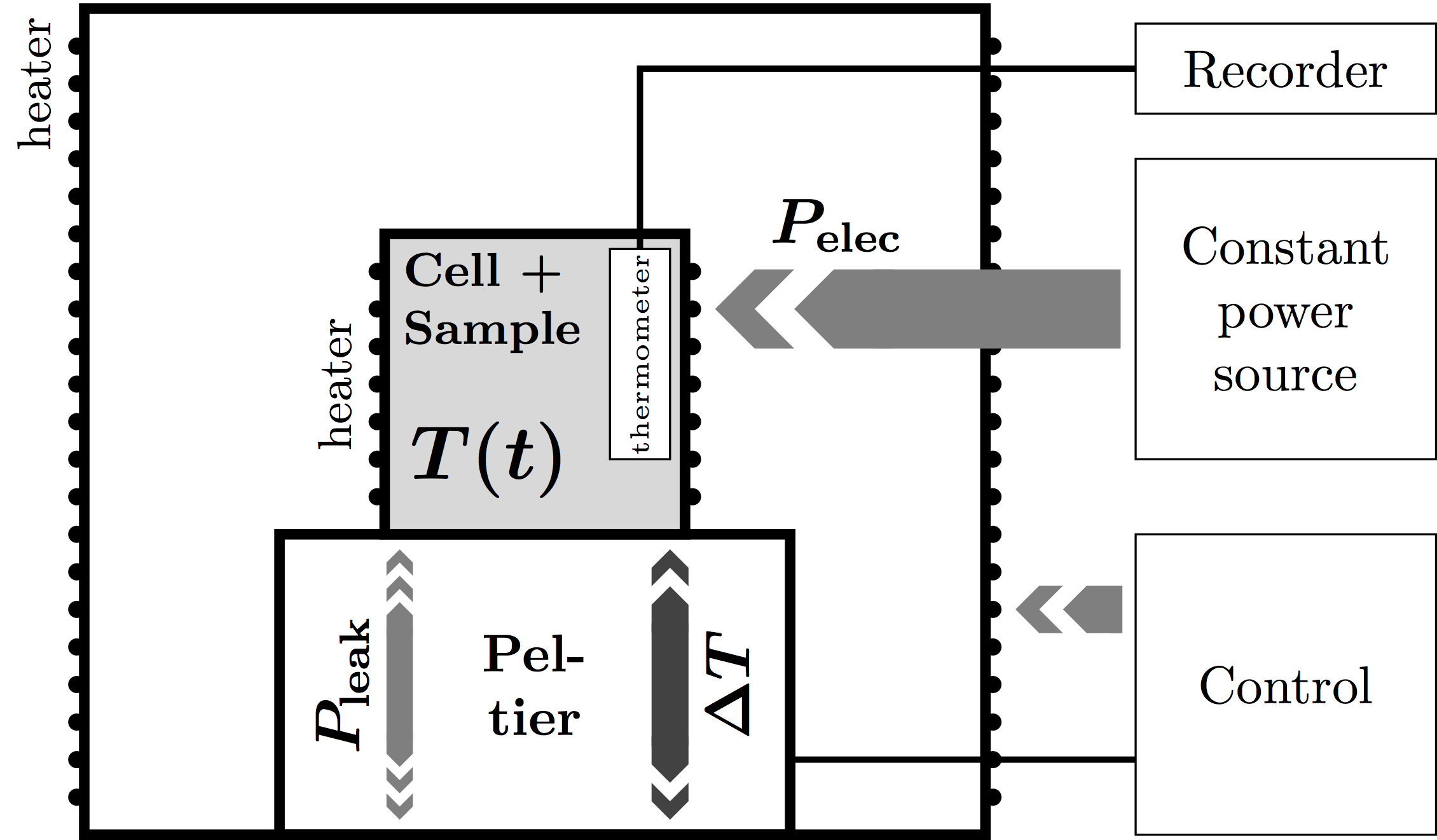
The Peltier-element-based ASC consists of an adiabatic shield, inside of which the sample holder is placed on top of a Peltier element. A constant power is applied to the sample, causing the sample to heat up. The Peltier element detects the temperature difference and instructs the control system to bring this difference to zero. Meanwhile, the Peltier element records any residual heat leak for further correction.
From these data, the heat capacity and enthalpy can be calculated through
$$m c_P + C_{bg} = P/{{dT}/{dt}} = P/T↖{.}$$
$$m h + H_{bg} = P t$$
The background values have been obtained from a factory-performed calibration experiment.
A pASC implementation
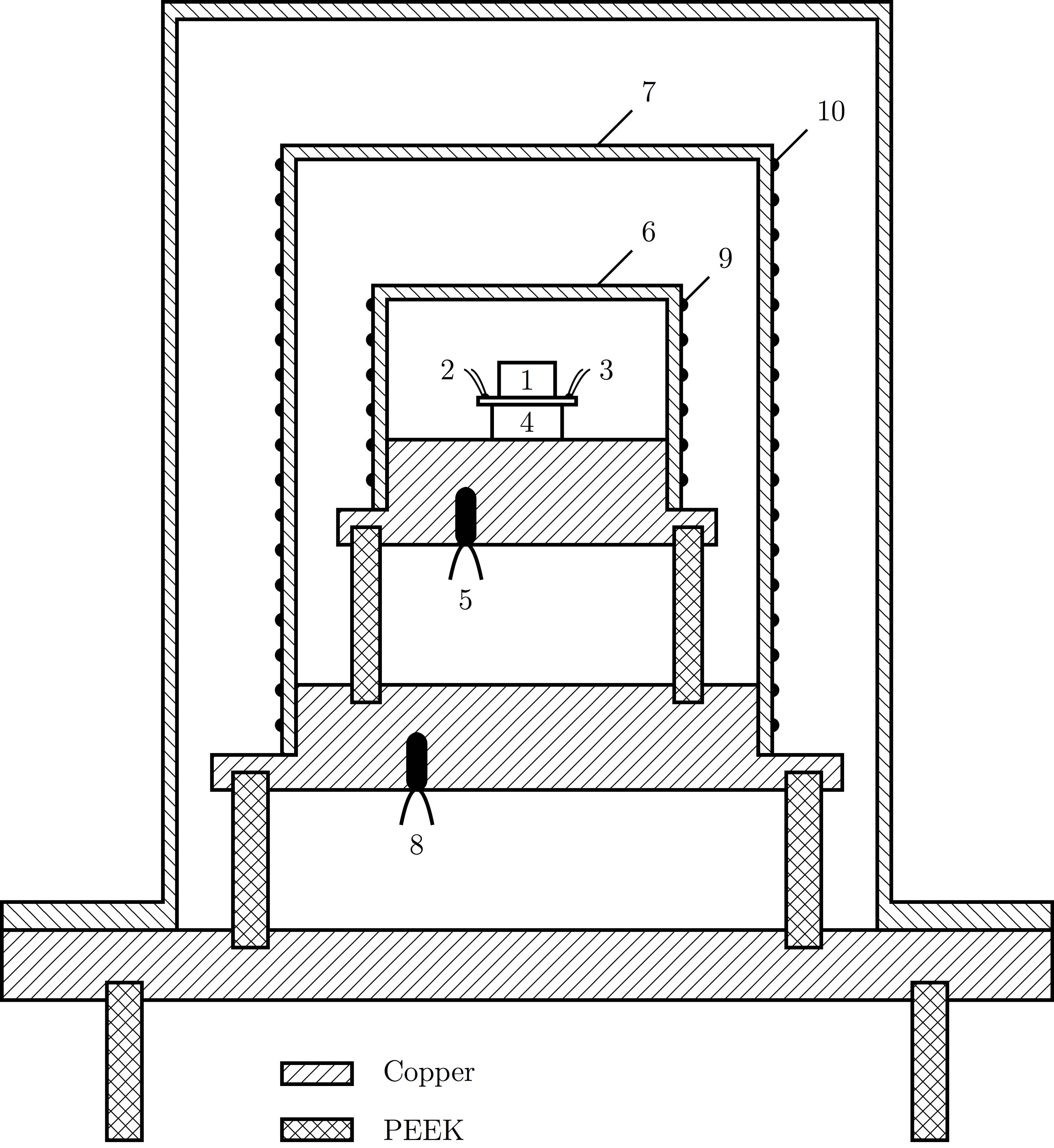
The sample cell (1) is placed on the sample holder, equipped with a thermometer (2) and a heater (3). The sample holder is fixed on top of the Peltier element (4). The element, in turn, is soldered on top of the adiabatic shield (6), which has it own thermometer (5) and heater (9). This is the center of calorimeter, and implements the pASC concept as explained above.
This "calorimeter core" is surrounded by a second, thermal shield (7), again with a thermometer (8) and heater (10). Around is the external jacket, which acts both a vacuum jacket and as a heat bath. It allows the calorimeter to run in vacuum if desired, and the temperature range of the bath determines the operational temperature range of the calorimeter. These additional shields are added to provide a controlled environment inside of which the calorimeter core operates
The calorimeter as depicted here is about 20 cm high. It is placed inside a temperature chamber with a range between -60 °C and 150 °C.
Other operation modes
Heat-flux constant rate mode
The Peltier element, which is used as a differential temperature sensor in the default ASC mode, can also be used to measure heat flux between the sample holder and the adiabatic shield. In the heat-flux operation mode, the adiabatic shield is heated up or cooled down at a constant temperature rate. The sample holder must follow this temperature evolution, and a heat flux between the sample holder and the adiabatic shield emerges, through the Peltier element. Since the Peltier element can measure heat flux, the variable power input to the sample holder is recorded, and the heat capacity can be evaluated.
Power-compensated constant rate mode
Because a heater is present on the sample holder, a mode of operation similar to the working principle of power-compensated DSCs is also possible. The adiabatic shield is heated or cooled at a constant rate, and the power that is needed to make the sample follow the shield is provided by the sample heater. Any residual heat leak that is not compensated by the sample heater is tracked by the Peltier element and can be used as a correction term. With the power and rate known, the heat capacity can be calculated.
Heat-step mode
In heat-step mode, an ASC acts the same way as classical heat-step calorimeter, also know as a Nernst calorimeter or an adiabatic calorimeter. First, the calorimeter is allowed to stabilise at a given temperature. Then, a short heat pulse is provided to the sample holder, which consequently heats up. After some time, a new equilibrium temperature is reached. From the height of the heat step and amount of heat involved, the heat capacity can be calculated. After sufficient stabilisation at the new temperature, a new heat step can be made.